Fexofenadine a First-Line Option for Seasonal Allergic Rhinitis and Chronic Idiopathic Urticaria
In Brief and Introduction
Fexofenadine, the active metabolite of terfenadine, is a selective histamine H1 receptor antagonist that is effective in the alleviation of symptoms associated with seasonal allergic rhinitis and chronic idiopathic urticaria. Like other nonsedating H1 receptor antagonists such as Loratidine and Cetirizine, Fexofenadine reduces sneezing, rhinorrhoea, itchy nose, palate or throat, and itchy, watery, red eyes in adults and adolescents with seasonal allergic rhinitis. Compared with placebo, once daily fexofenadine reduced the symptoms of chronic idiopathic urticaria for up to 6 weeks. Importantly, it appears that fexofenadine is free of adverse cardiac events; it has not been associated with prolongation of the QTc interval or torsades de pointes. Evidence indicates that there is no cognitive or psychomotor impairment with fexofenadine at dosages up to 240 mg/day.
Urticaria (hives) is characterised by transient erythema and wheals on the skin, often accompanied by intense itching. Episodes of urticaria that last for more than 6 weeks are considered chronic. Most cases of chronic urticaria are idiopathic; an exact external cause cannot be determined.[1] Symptoms of chronic idiopathic urticaria are thought to result from activation of mast cells in the skin with subsequent histamine release.[1]
Allergic rhinitis is characterised by the temporal relationship of symptoms to allergen exposure. Allergens can include animal dander, house dust mites and mould. Allergic rhinitis may be classified as seasonal allergic rhinitis if symptoms are pollen-induced. The natural history of seasonal allergic rhinitis is marked by acute exacerbations, especially when pollen counts are high, and remissions.
Seasonal allergic rhinitis, or hay fever, is an inflammatory disorder whose symptoms are mediated by the release of histamine. It is characterised by 1 or more of the following symptoms:[2]
- Sneezing
- Nasal congestion
- Itching of the nose
- Rhinorrhoea
- Postnasal drainage
- Ocular symptoms (itchy, red, watery eyes)
- Ear symptoms (congestion, popping, reduced hearing, pain)
- Sinus symptoms (sinus pressure or pain, headaches)
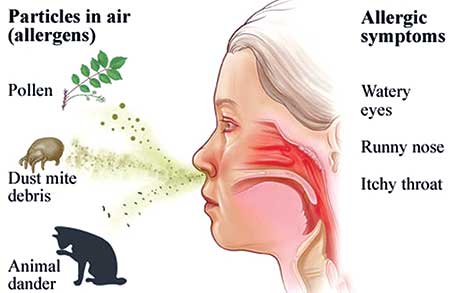
Photo: Internet
Fexofenadine a First-Line Option...
The nonsedating histamine H1 receptor antagonists, such as fexofenadine, are the mainstay of therapy for seasonal allergic rhinitis and chronic idiopathic urticaria (see Differential features table).[1,4] Prophylactic use of an antihistamine at the beginning of the pollen season or 2 to 5 hours prior to allergen exposure will enhance benefits in seasonal allergic rhinitis.[4] In the treatment of chronic idiopathic urticaria, nonsedating antihistamines are valuable when administered on a routine basis, as these agents are unable to displace histamine once it has bound to the receptor.
Fexofenadine is the active metabolite of terfenadine. It is a selective histamine H1 receptor antagonist that does not cross the blood brain barrier; therefore, it is not associated with sedation or adverse cognitive effects.[6]Unlike older antihistamines, fexofenadine does not have anticholinergic activity.[6]
Fexofenadine appears to have anti-inflammatory activity and has demonstrated inhibitory effects on chemical mediators (e.g. cytokines, leukotrienes and adhesion molecules) of the early phase response to intranasal allergen challenge.[3] These effects were seen in vitro at concentrations approximating those achieved with clinical dosages.[6]

Photo: Internet
Fexofenadine is rapidly absorbed, has an onset of action ranging from 1 to 3 hours and a long elimination half-life (11-14 hours).[6] It relieves the symptoms of allergic rhinitis, such as itching, sneezing, rhinorrhoea and itchy, watery, red eyes.[6] Importantly, 2 weeks' treatment with fexofenadine administered once (120 or 180mg) or twice (40 to 240mg) daily showed greater improvement in symptom scores than placebo, indicating 24-hour effectiveness.[6] Fexofenadine dosages of ≥20mg twice daily and 180mg once daily demonstrated significant improvements in mean pruritus severity scores compared with placebo in 2 double-blind trials in patients with chronic idiopathic urticaria.[6]
Fexofenadine ≥120 mg/day was generally as effective as cetirizine 10 mg/day and loratidine 10 mg/day in reducing the symptoms of seasonal allergic rhinitis after 1 to 2 weeks' treatment.[7,8] In 1 study, fexofenadine, but not loratidine or placebo, significantly relieved itchy, watery, red eyes; other symptoms were equally improved by both active agents compared with placebo.[9] There have been no comparative trials with fexofenadine and the intranasal nonsedating antihistamines (azelastine and levocabastine) in patients with seasonal allergic rhinitis.
As with other oral nonsedating antihistamines, fexofenadine has little effect on nasal congestion. The combination of fexofenadine with the decongestant pseudoephedrine was more effective than either agent alone in patients with seasonal allergic rhinitis and severe nasal congestion.[14]
Quality of Life Improved
As assessed by patients with chronic idiopathic urticaria, fexofenadine ≥60 mg/day significantly reduced disease interference with normal daily activities and sleep; quality of life was judged significantly better compared with placebo.[6] In comparative trials, fexofenadine 120 mg/day showed greater improvement in quality of life than loratidine 10 mg/day and provided greater quality of life when added to pseudoephedrine.[6] In preliminary reports from 2 multicentre trials, fexofenadine 60mg twice daily improved quality of life in patients with chronic idiopathic urticaria after 4 weeks' treatment.[6]
Excellent Tolerability Profile
Fexofenadine appears to have a tolerability profile that is generally similar to the other nonsedating antihistamines that are listed in the Differential features table. The most common adverse effects include headache, fatigue, drowsiness and nausea. Fexofenadine is unlikely to cause sedation in most individuals; dosages up to 240 mg/day appear to lack the sedative effects of older antihistamines.[6] Nevertheless, some patients may be more sensitive to the effects of the drug which must be determined in each individual patient.
In randomised, placebo-controlled clinical trials there were no significant cardiac events, including prolongation of the cardiac QTc interval, in patients who received therapeutic dosages of fexofenadine.[6] In clinical trials involving >6000 patients, no episodes of torsades de pointes have been observed.[6]
However, there has been a case report of QTc prolongation, polymorphic ventricular tachycardia and fibrillation with fexofenadine.[6] Therefore, the use of fexofenadine in susceptible patients or in combination with other agents that may increase the arrhythmogenic potential of the drug should be avoided.
Prescribing and Formulary Considerations
Although efficacy appears to be equal among the newer agents in the treatment of mild to moderate seasonal allergic rhinitis and chronic idiopathic urticaria, response to different antihistamines varies between individuals. The choice of agent is likely to be based on cost, administration interval, dosage form, availability, patient choice and adverse event profile.
Fexofenadine is preferred over older antihistamines because of its apparent lack of sedation, cognitive/ psychomotor impairment and anticholinergic effects that are common among the older agents. Tolerance does not appear to develop with long term use of fexofenadine in chronic idiopathic urticaria. In addition to its lack of hepatic metabolism, these reasons make fexofenadine attractive for older patients. The higher cost of newer compared with older antihistamines may be justified in elderly patients because of their relative lack of adverse effects, particularly sedation and drowsiness, which may lead to falls.[5]
Source: https://www.medscape.com/viewarticle/406437
References:
[1] Kenedy MS. Evaluation of chronic eczema and urticaria and angioedema. Immunol Allergy Clin North Am 1999; 19 (1): 19-33.
[2] Storms WW. A comprehensive diagnostic approach to upper airway disease. J Allergy Clin Immunol 1998; 101: S361-3.
[3] Slater JW, Zechnich AD, Haxby DG. Second-Generation anti-histamines: A comparative review. Drugs 1999 Jan; 57 (1) 31-47.
[4] Dykewwicz MS, Fineman S, Skoner DP, et al. Diagnosis and management of rhinitis: complete guildlines of the Join Task Force on Practice Parameter in Allergy, Asthma and Immonology. Ann Allergy Asthma Inmmunol 1998 Nov; 81 (Pt 2): 478-518.
[5] Britisj National Formulary No 39. London: The Pharmaceutical Press 2000 Mar: 149-50, 490.
[6] Simpson K, Jarvis B. Fexofenadin: a review of its use in the management if seasonal allergic rhinitis and chronic idiopathic urticaria. Drugs 2000 Feb; 59 (2): 301-21.
[7] Howarth PH, Stern MA, Roi L, et al. Double blind, placebo-controlled study comparing the efficacy and safety of Fexofenadine hydrocloride (120 and 180mg once daily) anf cetirizin in seasonnal Allergic rhitnitis. J Allergy Clin inmmunol 1999; 104 (5): 927-33.
[8] Kaiser H, Harris AG, Capano D, et al. A double-blind, placebo-controlled comparison off the safety and efficacy of Loratadin, Fexofenadin HCl and placebo in th treatment of subjects with seasonal allergic rhinitis [abstract no P322]. Allergy 1999; 54 Suppl. 52: 155.
[9] Van Cauwenberge P, Juniper EF, Meltzer E, et al. Efficacy, safety and quality of life – a comparison between fexofenadine, loratadine, and placebo in a treatment of seasonal allergic rhinitis. Presented at the American College of Allergy, Asthma and Immunonlogy Conference, 1999 Nov; Chicago.
[10] Sussman GL, Mason J, Compton D, et al. The efficacy and safety of fexofenadine HCl and pseudoephedrine, alone and in combination, in seasonal allergic rhinitis. J Allergy Clin Inmmunol 1999; 104 (1): 100-6.










